TIM-3 therapy for Alzheimer’s has emerged as a promising avenue in the fight against this devastating disease. Recent studies reveal that by targeting the TIM-3 checkpoint molecule, which is known to inhibit the immune function of microglia in the brain, there may be potential to improve cognitive abilities in Alzheimer’s patients. This innovative approach leverages the understanding of immune system strategies traditionally used in cancer therapy, bringing new hope to those affected by Alzheimer’s. By unlocking the power of microglia, researchers aim to enhance their ability to clear amyloid plaques that plague the brains of individuals with Alzheimer’s disease. As TIM-3 gene research progresses, the prospects for effective Alzheimer’s treatment are becoming more tangible, paving the way for transformative therapies in the near future.
The exploration of TIM-3 therapy for Alzheimer’s disease presents a groundbreaking intersection of neuro-immunology and neurodegenerative research. As scientists delve into the mechanics of checkpoint molecules, they uncover their crucial role not only in regulating immune responses but also in influencing microglial behavior in the aging brain. This fascinating approach posits that alleviating the inhibitory effects of TIM-3 could activate microglia, enabling these brain-resident immune cells to effectively remove harmful plaque deposits and improve memory function. By harnessing what we know from cancer therapy, researchers are forging a new path toward innovative Alzheimer’s treatments, revolutionizing the landscape of cognitive health. The emerging evidence suggests that enhancing microglia function through TIM-3 inhibition could be a pivotal strategy in thwarting the progression of Alzheimer’s disease.
TIM-3 Therapy: A New Frontier in Alzheimer’s Treatment
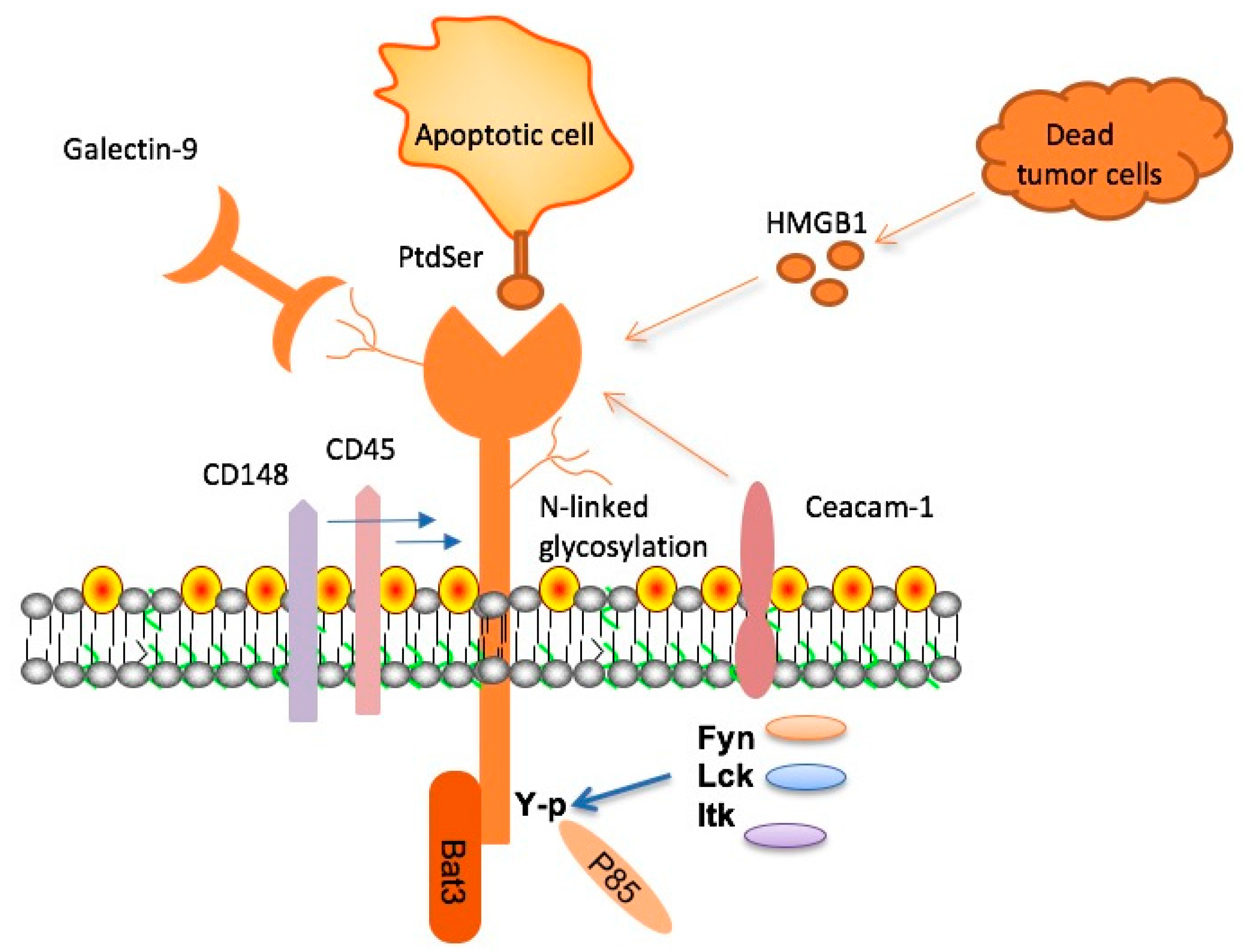
Recent research has unveiled the potential of TIM-3 therapy as a revolutionary approach in Alzheimer’s treatment. This therapy leverages the concept of checkpoint molecules, which have historically been successful in cancer therapies. TIM-3, an inhibitory checkpoint molecule, plays a crucial role in regulating microglial activity in the brain. By deleting TIM-3 from microglia, researchers found that these immune cells could effectively engage and clear amyloid plaques, restoring cognitive functions in affected models. This discovery opens up new pathways for therapeutic interventions that target the underlying mechanisms of Alzheimer’s disease.
The significance of TIM-3’s role in Alzheimer’s treatment is further underscored by genetic research linking the TIM-3 gene to late-onset Alzheimer’s. Variations in the HAVCR2 gene, which encodes TIM-3, have been associated with increased Alzheimer’s risk. By inhibiting or blocking TIM-3, it is possible to unleash microglia from their homeostatic state, allowing them to clear the amyloid and tau deposits that characterize the disease. As Alzheimer’s treatment continues to evolve, TIM-3 therapy stands at the forefront, promising a more effective strategy against neurodegeneration.
Understanding Checkpoint Molecules in Alzheimer’s Disease
Checkpoint molecules are integral components of the immune system, acting to prevent overactivation during immune responses. In the context of Alzheimer’s disease, their role becomes particularly crucial. TIM-3, being one such molecule, inhibits the ability of microglial cells to clear damaging amyloid plaques. This inhibition leads to an accumulation of plaques, which is a hallmark of Alzheimer’s pathology. Understanding the dynamics of checkpoint molecules is essential for developing new treatment modalities that can manipulate these pathways to enhance immune function in the brain.
The study of TIM-3 highlights a dual role for checkpoint molecules in both cancer and Alzheimer’s treatment. While they serve protective functions in preventing autoimmunity, their overexpression can contribute to the progression of neurodegenerative diseases. By focusing on how these molecules can be targeted or modified, researchers are reevaluating approaches to Alzheimer’s treatment that could lead to innovative therapies aimed at restoring microglial function and promoting plaque clearance.
Microglia’s Crucial Role in Alzheimer’s Pathophysiology
Microglia are the primary immune cells of the brain, essential for maintaining homeostasis, supporting neuronal health, and responding to injury. In Alzheimer’s disease, however, the function of microglia becomes compromised. The heightened expression of TIM-3 in aging microglia prevents these cells from effectively clearing amyloid plaques, leading to a vicious cycle of inflammation and cognitive decline. Understanding the immune dynamics at play enables researchers to explore treatments that can restore normal microglial function.
Research indicates that once microglia adopt a homeostatic state due to TIM-3 overexpression, their ability to perform essential tasks, such as synapse pruning and debris clearance, is significantly impaired. This dysfunction is detrimental, as it contributes to the pathogenesis of Alzheimer’s disease. Investigating strategies to reprogram microglia, such as TIM-3 inhibition, may offer new avenues for alleviating cognitive symptoms and improving patient outcomes.
The Intersection of Cancer Therapy and Alzheimer’s Research
The successful application of cancer treatment strategies to Alzheimer’s research marks an exciting cross-disciplinary approach that leverages existing knowledge about immune regulation. By understanding how TIM-3 functions in cancer therapy—inhibiting immune responses to allow tumor survival—researchers are repurposing this insight to combat Alzheimer’s, a disease that similarly involves immune dysfunction. This intersection could expedite the development of effective treatments by integrating methodologies from various fields.
The exploration of TIM-3 therapy showcases the potential benefits of applying cancer immunotherapy principles to neurodegenerative conditions. Given that many drugs have failed in Alzheimer’s trials, adapting existing anti-TIM-3 antibodies could lead to novel therapeutic options with fewer side effects. The research encourages a paradigm shift in how Alzheimer’s is conceptualized, paving the way for targeted immune therapies that could improve patient care significantly.
Innovations in Genetic Research: The TIM-3 Gene
Genetic research plays a pivotal role in understanding Alzheimer’s disease, particularly in elucidating the impacts of the TIM-3 gene. Studies have established a link between polymorphisms in the TIM-3 gene (HAVCR2) and a higher genetic risk for late-onset Alzheimer’s. By investigating how variations in this gene affect microglial function and amyloid clearance, researchers are gaining insights into individual susceptibility to the disease, which could inform personalized treatment strategies.
Furthermore, innovations in genetic engineering have allowed scientists to create mouse models that can help simulate human disease pathology. By deleting the TIM-3 gene in these models, researchers noted pronounced improvements in cognitive abilities and plaque clearance. These advancements signify a crucial step in translational research, aiming to develop gene-targeted therapies that may one day replace ineffective treatment methodologies.
Next Steps In Alzheimer’s Research: Investigating Human Applications
Following promising results in laboratory mice, the next critical phase in Alzheimer’s research involves translating these findings to human applications. Researchers are currently investigating whether human anti-TIM-3 antibodies can successfully inhibit plaque development in Alzheimer’s disease models. If successful, this would represent a significant leap towards fostering effective treatments for patients while the focus remains on minimizing potential adverse effects.
The application of TIM-3 therapy in humans could involve various approaches, such as monoclonal antibodies or small molecule inhibitors targeting TIM-3 functions. As researchers progress towards clinical trials, the emphasis will be on understanding how TIM-3 modulation can be tailored to the unique characteristics of Alzheimer’s patients, making it imperative to consider patient-specific factors to enhance treatment efficacy.
Challenges in Alzheimer’s Treatment Development
Despite advancements in understanding Alzheimer’s disease, developing effective treatments remains a significant challenge. The complexities associated with amyloid beta clearance, along with the intricacies of the brain’s immune system functions, complicate the design of effective interventions. The inadequacy of current anti-amyloid therapies, which often fail to bring about substantial improvement or lead to unwanted side effects, highlights the need for innovative strategies like TIM-3 targeting that address the disease’s root causes.
Moreover, the failure of numerous high-profile drug trials underscores the importance of rigorous research methodologies. Identifying specific biomarkers related to TIM-3 expression and its interactions with microglia could help stratify patient populations for future studies. Such detailed insights will be essential in navigating the roadblocks in Alzheimer’s research and ensuring that upcoming therapies demonstrate tangible benefits.
Regulatory Considerations for TIM-3 Therapy
As scientists advance towards clinical applications of TIM-3 therapy for Alzheimer’s, regulatory considerations come to the forefront. Any drug that alters immune checkpoint activity must undergo thorough examination to assess both safety and efficacy in human populations. Regulatory agencies will require robust clinical data demonstrating that manipulating TIM-3 can yield significant cognitive improvements without imposing undue risks.
Additionally, researchers will need to navigate the challenges of trial design, including appropriate endpoints that reflect meaningful changes, especially in cognitive and functional assessments. Ongoing dialogue with regulatory bodies will be crucial to accelerate bringing TIM-3 therapies from bench to bedside while ensuring adherence to safety protocols.
The Future of Alzheimer’s Therapeutics: Integrating Immune Responses
Looking ahead, the future of Alzheimer’s therapeutics seems to hinge on a comprehensive understanding of immune responses in the brain. The innovative application of therapies targeting TIM-3 could serve as a model for integrating immune system modulation into treatment paradigms. As researchers continue to unveil the complex interplay between immune checkpoint molecules and neurodegeneration, the prospect of achieving better cognitive outcomes becomes increasingly feasible.
This forward-thinking approach suggests a need for collaborative efforts across various scientific disciplines to optimize treatment efficacy. By fusing insights from immunology, genetics, and neurobiology, researchers can pioneer transformative therapies that not only address plaques but also restore overall cognitive function and quality of life for individuals afflicted by Alzheimer’s.
Frequently Asked Questions
What is TIM-3 therapy for Alzheimer’s and how does it work?
TIM-3 therapy for Alzheimer’s focuses on inhibiting the TIM-3 checkpoint molecule that prevents microglial cells from clearing amyloid plaques in the brain. By blocking TIM-3, research shows that these immune cells can effectively attack and remove plaques, potentially improving cognitive functions and memory in Alzheimer’s patients.
How does TIM-3 function as a checkpoint molecule in Alzheimer’s treatment?
In Alzheimer’s treatment, TIM-3 functions as an inhibitory checkpoint molecule that limits the activity of microglia, the brain’s immune cells. High levels of TIM-3 prevent microglia from engulfing amyloid plaques, which contribute to cognitive decline. Targeting TIM-3 may enhance microglial function and aid in plaque clearance.
What are the potential benefits of TIM-3 therapy for Alzheimer’s patients?
The potential benefits of TIM-3 therapy for Alzheimer’s patients include improved memory and cognitive abilities. Studies suggest that blocking TIM-3 can restore the ability of microglia to clear amyloid plaques, thereby alleviating some symptoms of Alzheimer’s disease and enhancing overall brain health.
Is TIM-3 only related to Alzheimer’s, or does it play a role in other conditions?
While TIM-3 is prominently featured in Alzheimer’s treatment, it also has implications in cancer therapy as an immune checkpoint molecule. Its ability to regulate immune responses makes TIM-3 a focal point in understanding various diseases, including autoimmune conditions and cancer, highlighting its versatility in therapeutic strategies.
How does the TIM-3 gene polymorphism relate to Alzheimer’s disease risk?
The TIM-3 gene, specifically the HAVCR2 polymorphism, has been linked to an increased genetic risk for late-onset Alzheimer’s disease. This polymorphism is associated with higher expression of TIM-3 in microglia, impairing their ability to clear amyloid plaques, which is crucial for Alzheimer’s pathology.
What are the next steps for TIM-3 therapy research in Alzheimer’s treatment?
Current research is focusing on testing human anti-TIM-3 antibodies in mouse models of Alzheimer’s. These studies aim to examine the effectiveness of blocking TIM-3 to inhibit plaque formation and possibly restore cognitive functions, paving the way for potential clinical trials in humans.
Can TIM-3 therapy for Alzheimer’s be combined with existing treatments?
Yes, TIM-3 therapy for Alzheimer’s has the potential to be combined with existing treatments, particularly those targeting amyloid beta. By enhancing microglial function and reducing plaques through TIM-3 inhibition, patients may experience synergistic effects that improve drug effectiveness and overall disease management.
What kind of research has been conducted on TIM-3 therapy for Alzheimer’s?
Research on TIM-3 therapy for Alzheimer’s has demonstrated that genetically deleting the TIM-3 gene in experimental mouse models leads to improved plaque clearance and cognitive function. Studies have highlighted its role in modulating immune cell activity, paving the way for innovative therapeutic strategies.
What challenges exist in developing TIM-3 therapy for Alzheimer’s?
Challenges in developing TIM-3 therapy for Alzheimer’s include ensuring that treatments selectively target the brain’s immune cells without affecting peripheral immune responses, as well as addressing the complexities of human brain biology compared to mouse models. Ongoing research aims to address these obstacles.
How might TIM-3 therapy change the future of Alzheimer’s disease treatment?
TIM-3 therapy has the potential to revolutionize Alzheimer’s treatment by offering a new mechanism to enhance the brain’s immune response against plaque accumulation. This approach could lead to more effective interventions, ultimately improving patients’ quality of life and cognitive function.
| Key Point | Details |
|---|---|
| Purpose of Study | Exploring TIM-3 therapy for Alzheimer’s based on its success in cancer treatment. |
| Role of TIM-3 | TIM-3 inhibits microglial action against amyloid plaques in the brain. |
| Microglial Cells | The immune cells of the brain that clear plaques but are inhibited by TIM-3. |
| Research Findings | Mice with deleted TIM-3 showed improved plaque clearance and cognitive function. |
| Potential Therapy | Could involve anti-TIM-3 antibodies or molecules to block TIM-3’s function. |
| Future Directions | Testing human anti-TIM-3 in Alzheimer’s mouse models for efficacy. |
Summary
TIM-3 therapy for Alzheimer’s presents a promising new approach in the fight against this neurodegenerative disease. By targeting the TIM-3 molecule, researchers have discovered a possible way to enhance the brain’s immune response to clear harmful amyloid plaques. The findings from studies on mice show not only increased memory function but also a potential pathway for developing therapies that could be translated into human treatment. This innovative approach could shift the Alzheimer’s treatment landscape, emphasizing the importance of immune regulation in brain health.