Patient safety in medical research is a critical concern that underscores the importance of ethical oversight and regulatory compliance. With the halt in funding for various research initiatives, particularly affecting the SMART IRB program, researchers face unprecedented challenges in ensuring that participant rights and safety remain paramount. The intersection of research funding impacts and medical research ethics has never been more relevant, as inadequate IRB oversight can jeopardize clinical trial protection measures essential for safeguarding patients. As federal research grants become increasingly limited, the ramifications ripple through the entire medical research landscape, affecting not only scientists but also the individuals relying on advancements in healthcare. Ultimately, prioritizing patient safety in medical research is essential for maintaining public trust and fostering robust scientific progress.
Ensuring the well-being of individuals involved in clinical studies represents a fundamental aspect of medical inquiry. The ethical frameworks that guide research practices play a pivotal role in protecting participants, particularly in light of recent funding disruptions that threaten to undermine these protections. These challenges highlight the necessity of robust institutional mechanisms, such as oversight boards dedicated to safeguarding the interests of research subjects. Additionally, the ethics of medical investigation demand a vigilant commitment to transparency and responsibility, especially regarding the management of federal resources earmarked for such purposes. As we explore the implications of budgeting decisions on research infrastructures, the overarching commitment to participant welfare must remain at the forefront of our efforts.
Understanding the Impact of Research Funding Cuts
The halt in federal research funding, particularly the $2 billion freeze directed at institutions like Harvard, poses grave challenges to medical research. This disruption can lead to a cascading effect on numerous studies, especially those focused on safeguarding patient safety in medical research. Federal grants have historically underpinned significant projects aimed at protecting participant rights and ensuring the ethical advancement of clinical practices. When funding ceases, researchers face immediate threats to their ongoing projects, leading to suspensions of studies that rely heavily on continuous oversight to assure participant welfare.
The implications of research funding cuts extend far beyond immediate logistical issues. With research collaborations stunted, innovations in patient care can stall indefinitely, risking delays in the discovery of essential treatments and medical advancements. The integrity of clinical trials is at stake, as patients may not only lose access to cutting-edge treatments but may also lose confidence in the systems designed to protect them. Fostering trust is critical; a break in this chain due to funding insufficiency could discourage future participation in vital research, further compounding the crisis.
Frequently Asked Questions
How does patient safety in medical research benefit from IRB oversight?
Patient safety in medical research is significantly enhanced through the oversight provided by Institutional Review Boards (IRBs). These boards review research proposals to ensure that they comply with ethical standards and protect the rights of participants. IRBs assess risk levels, ensure informed consent, and monitor the safety measures implemented throughout clinical trials, thus acting as a critical line of defense against potential harms to patients.
What impact does research funding have on patient safety in medical research?
Research funding plays a crucial role in maintaining patient safety in medical research. Adequate funding enables institutions to implement robust IRB oversight, conduct comprehensive safety assessments, and train researchers in medically ethical practices. Cuts to federal research grants can disrupt these safety measures, leading to potential risks for patients involved in clinical trials due to halted research and less stringent oversight.
Why is medical research ethics essential for patient safety?
Medical research ethics is foundational to ensuring patient safety in clinical trials. Ethical guidelines govern the treatment of research participants, focusing on informed consent, risk minimization, and the welfare of patients. Adhering to these principles helps to protect individuals from exploitation, harm, or deception during research activities, thereby reinforcing public trust in the medical research process.
How do federal research grants support clinical trial protection for patients?
Federal research grants provide essential funding that supports clinical trial protection for patients by enabling institutions to maintain stringent oversight through IRBs. These grants assist in implementing safety protocols, monitoring ongoing trials for adverse events, and ensuring compliance with ethical research standards, all contributing to safeguarding the health and well-being of participants in medical studies.
What challenges arise from a halt in funding concerning patient safety in medical research?
A halt in funding presents significant challenges to patient safety in medical research. It can lead to disruptions in ongoing studies, decreased IRB oversight, and delays in implementing safety protocols. These disruptions may ultimately compromise the safety of research participants, deepen skepticism toward clinical research, and hinder the advancement of necessary medical innovations.
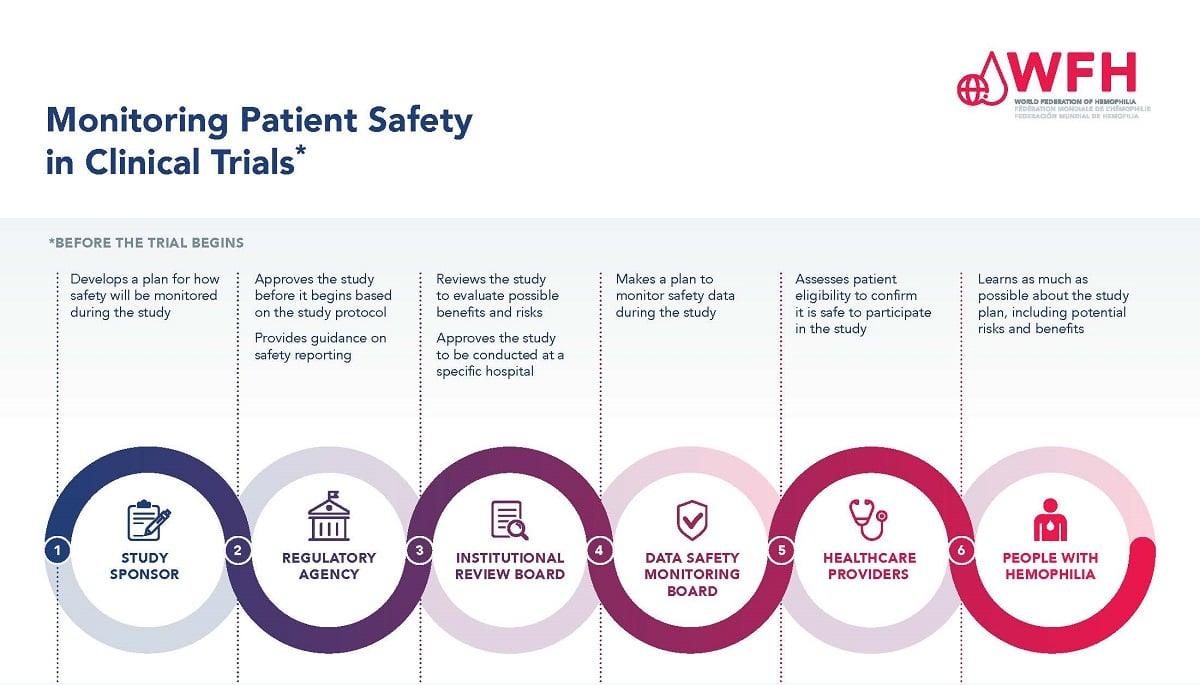
How does the SMART IRB system enhance oversight in medical research to ensure patient safety?
The SMART IRB system enhances oversight in medical research by facilitating a streamlined review process across multiple sites, thereby ensuring consistent patient safety measures. By having a single IRB oversee collaborative studies, this system reduces approval delays and minimizes the administrative burden, allowing quicker implementation of studies that adhere to ethical standards protecting participant welfare.
| Key Points |
|---|
| The freeze on research funding by the Trump administration has stalled patient safety improvements in medical research. |
| SMART IRB is a national system that facilitates oversight of multi-site medical research, ensuring compliance and protection of patient rights. |
| IRBs are essential for reviewing research proposals to safeguard participant rights and welfare through ethical oversight. |
| Cutbacks in funding can lead to halted research, reinforcing public mistrust and potentially putting participants at risk. |
| The ongoing support from institutions is crucial to maintain safety measures and collaborative research despite funding challenges. |
Summary
Patient safety in medical research is critically affected by funding, as halted grants disrupt essential oversight mechanisms. The freeze on federal research funds has stymied efforts to ensure the safety and rights of participants in clinical studies, exemplifying how funding decisions can jeopardize public trust and participant welfare. Ensuring patient safety in medical research requires continuous support and funding to maintain rigorous ethical standards and effective oversight through Institutional Review Boards (IRBs). Without adequate funding, the potential risks to participants and the integrity of the entire research process are heightened.