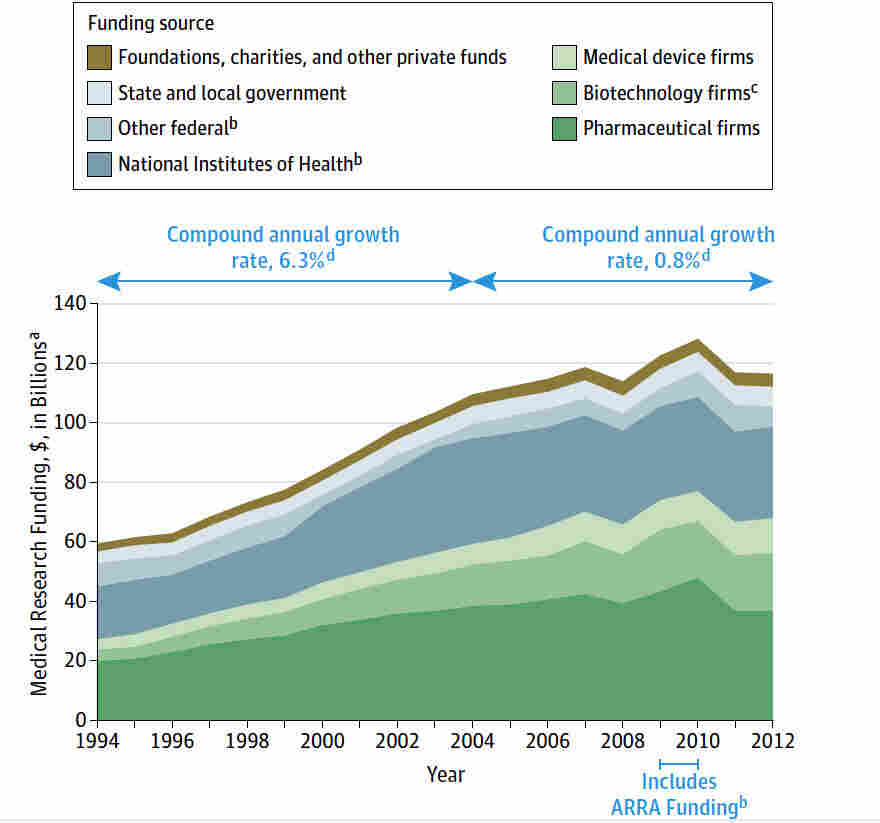
Medical research funding is critical in ensuring the effective oversight and ethical conduct of research aimed at improving patient safety. Recent funding cuts, particularly to NIH research grants, have raised concerns about the implications for patient welfare and the integrity of medical studies. These financial constraints hinder the necessary Institutional Review Board (IRB) oversight, which is essential for maintaining ethical standards in research involving human subjects. As funding diminishes, the impact on research is profound, potentially leading to compromised patient safety and eroding trust within communities that participate in clinical trials. Addressing the “impact of funding cuts on research” is crucial to safeguard participant rights, uphold medical research ethics, and advance the science that benefits us all.
The current landscape of financing clinical studies plays a pivotal role in shaping the future of healthcare research. With dwindling sources of financial support, various stakeholders are increasingly concerned about how these changes affect patient welfare and the ethical frameworks that govern medical investigations. Rigorous oversight mechanisms, including Institutional Review Boards (IRBs), are more vital than ever, as they ensure compliance with ethical guidelines while protecting the rights of individuals participating in studies. Furthermore, the repercussions of reduced funding levels can lead to significant delays and disruptions in research processes, ultimately impacting advancements in patient care and safety. As we explore alternative pathways for funding, the relationship between financial resources and the integrity of medical research remains a topic of paramount importance.
The Critical Role of Medical Research Funding
Medical research funding plays an essential role in the continuous development and advancement of healthcare practices. Federal and private funding sources, such as NIH research grants, provide the necessary resources to support innovative studies that lead to new treatments, drugs, and technologies. However, recent cuts in funding, like the significant freeze imposed by the Trump administration, threaten to disrupt this crucial funding landscape, resulting in halted research projects that could benefit countless patients. The immediate effects of such funding interruptions are profound, stalling collaborative efforts and delaying the investigation of new medical breakthroughs essential for treating various diseases.
Moreover, financial support is not just important for initiating medical research; it is equally required for maintaining consistent oversight throughout the research lifecycle. Institutions depend on this funding to ensure compliance with medical research ethics and to support institutional review boards (IRBs) that monitor patient safety throughout their trials. A consistent influx of medical research financing bolsters efforts to uphold rigorous protocols that safeguard the rights and welfare of patients participating in research. Without adequate funding, the risk of compromising these protective measures increases significantly.
Patient Safety in Research: A Financial Perspective
Ensuring patient safety in research is a primary obligation of every investigator and institution involved in clinical trials. However, this commitment can only be achieved with sufficient financial backing. Patient safety is directly influenced by the level of oversight and monitoring afforded by IRBs, which become strained under budget constraints resulting from funding cuts. Research institutions may struggle to allocate resources towards adequately trained personnel and robust oversight mechanisms, leading to potential risks in the execution of clinical trials. This reality is particularly concerning in an environment where ethical issues, such as informed consent and risk assessment, must be meticulously addressed to prevent harm.
The linkage between patient safety and funding is evident when considering the implications of disrupted financial support. The recent halt in federal funding, particularly the loss of NIH grants, jeopardizes established safety protocols by limiting the ability of IRBs to function effectively. Studies that aim to protect vulnerable populations may be put on hold, diminishing the opportunity for researchers to recruit participants and conduct necessary follow-ups. Ultimately, this could lead to an erosion of public trust in clinical research, making patients hesitant to engage in future trials.
Understanding the Impact of Funding Cuts on Research
Funding cuts have a cascading effect on the entire medical research spectrum, primarily impacting the patients who volunteer for studies. When research grants are slashed or put on hold, the investigative teams responsible for overseeing trial protocols and patient safety face significant challenges. These teams can’t recruit new participants, often forcing them to halt ongoing studies, which jeopardizes both the integrity of the research and the well-being of the participants involved. Without financial backing, clinical trials may not only be delayed but also potentially terminated, leading to a wastage of resources and loss of precious scientific knowledge.
Additionally, the sociocultural implications of funding cuts must be acknowledged. The healthcare community, including the citizens who have a vested interest in the outcomes of research, feels the repercussions when significant projects are abruptly halted. The resulting gap in patient participation and innovation can instill a sense of skepticism and mistrust towards the research community, negatively impacting future studies. Establishing and maintaining healthcare advancements depends heavily on consistent funding to nurture both the ethical aspects of research and the safety of those involved.
The Role of Institutional Review Boards (IRBs) in Protecting Patients
Institutional Review Boards (IRBs) are fundamental to the ethical oversight of medical research, ensuring participant rights and safety are prioritized. These boards conduct thorough reviews of research proposals to assess various elements, including risk mitigation, informed consent processes, and participant recruitment strategies. However, the operational effectiveness of IRBs is contingent upon an adequate supply of funding. Budget cuts can lead to reduced staff training and resource allocations, compromising the IRB’s ability to perform its duties efficient and thoroughly. Fewer resources can result in longer review times and less comprehensive oversight, inadvertently putting participants at risk.
Furthermore, the societal importance of IRB oversight has become increasingly evident in the context of historical research abuses. The establishment of today’s ethical guidelines stems from past injustices in medical research. When funding is compromised, the integrity of these guidelines is similarly put at risk. It is crucial for researchers to uphold ethical research practices to avoid repeating mistakes from our past. Robust and well-funded IRB systems act as a safety net for participants, ultimately ensuring their rights and well-being remain protected.
Historically Significant Research and Its Ethical Oversight
The landscape of medical research has evolved significantly, influenced heavily by past ethical failures that have emphasized the need for stringent oversight. Historical abuses, such as the Tuskegee Study and the Willowbrook hepatitis trials, highlight the dire consequences of inadequate ethical regulations. These events led to the enactment of laws that emphasize informed consent and participant safety, thus instituting the IRB framework that governs modern research ethics. Continuing to uphold these ethical standards is not merely a bureaucratic requirement but a moral obligation that reflects society’s commitment to honoring individuals’ rights.
The financial aspects of maintaining ethical oversight are paramount in ensuring that research conducted today adheres to the high standards set by past tragedies. When sustenance for research oversight falters, the lessons of the past become easily overshadowed by the immediate challenges researchers face—leading to ethical compromises. Adequate funding is vital to sustain the infrastructure needed for thorough IRB reviews and participant protections, making it imperative that funding remains robust and accessible.
The Interconnection Between Funding and Collaborative Research
Collaborative research endeavors are essential for advancing innovative medical treatments and discoveries. Such large-scale projects often require input from multiple institutions, necessitating refined collaboration models that respect ethical standards and patient welfare. However, these collaborative efforts heavily rely on consistent funding streams, typically provided through federal grants such as those from the NIH. When funding cuts occur, these research collaborations face a multitude of challenges that can lead to delays and interruptions, ultimately affecting patient safety and research outcomes.
Moreover, the introduction of initiatives like the SMART IRB system is aimed at streamlining the ethics review process across all partner institutions, enhancing efficiency in collaborative trials. However, without adequate funding, the administrative aspects of maintaining such systems become severely impacted. Researchers may not have the resources necessary to navigate the bureaucratic processes of collaboration, which could lead to missed opportunities for critical advancements in treatment options for patients. Therefore, safeguarding funding is essential for fostering a collaborative environment capable of producing meaningful research outcomes.
The Significance of Ethical Conduct in Medical Research
Ethical conduct in medical research embodies the commitment to protect participants and enhance scientific integrity. The association between moral practices and adequate funding cannot be overstated; adherence to ethical standards requires resources to train staff, conduct oversight, and facilitate clear communication with participants. Without proper funding, research teams may find themselves under pressure to cut corners, which raises ethical concerns about participant risks, informed consent, and overall study transparency.
Furthermore, the implications of compromising ethical standards extend beyond immediate research outcomes; they can foster widespread distrust in the medical and research communities. A transparent and well-funded framework for ethical oversight, which includes effective IRB processes, is essential for reassuring stakeholders that their rights are respected during their participation in research. Maintaining ethical conduct hinges not only on researchers’ integrity but also on the environment that funding provides for conducting ethically sound research practices.
Public Trust: The Foundation of Medical Research
The success of medical research relies heavily on public trust, which can be easily eroded by funding cuts and transparency issues. Trust is built on the belief that participation in research offers a pathway to improving healthcare and that researchers prioritize participant safety and ethical conduct. Fluctuations in funding can create an atmosphere of uncertainty, leading many potential participants to question whether their involvement is truly valued within the larger research framework.
Moreover, strong community engagement is imperative for fostering a positive perception of clinical trials. When funding is disrupted, researchers may struggle to maintain their relationships with community stakeholders. This disconnect can lead to skepticism about the motivations behind research and the safety of trials, impeding recruitment efforts. Establishing a reliable flow of funding not only strengthens the research infrastructure but also rebuilds community trust, ensuring that participants feel secure and valued throughout the research process.
Investment in the Future of Medical Research
Investment in medical research is crucial for ensuring continuous improvements in patient care and therapeutic practices. Every dollar allocated towards research funding translates into potential lives saved, new treatments developed, and groundbreaking medical advancements. As governments and institutions face difficult budget decisions, prioritizing research funding becomes increasingly vital. The implications of this investment extend beyond individual institutions; they affect entire communities and the healthcare system as a whole, emphasizing the necessity of supporting robust research initiatives.
Ensuring a stable funding environment not only promotes patient safety but also encourages innovation and ethical conduct in research. Stakeholders, including government agencies, private corporations, and the public, must recognize the long-term benefits of supporting research endeavors. Investing in medical research equips institutions to tackle complex health issues and pursue ethical research that aligns with community values, ultimately driving the field of healthcare forward.
Frequently Asked Questions
How does medical research funding impact patient safety in research?
Medical research funding is crucial for maintaining patient safety in research. Adequate funding allows for thorough oversight by institutional review boards (IRBs), ensuring compliance with ethical standards that protect participants’ rights and welfare. Secure funding enables comprehensive training for research professionals, facilitating rigorous monitoring of study protocols, informed consent processes, and risk assessments, all essential for safeguarding patients in clinical trials.
What are the consequences of funding cuts on medical research ethics?
Funding cuts to medical research can significantly compromise research ethics. With reduced financial resources, institutions may struggle to maintain adequate IRB oversight, leading to lesser scrutiny of research proposals and potentially unethical practices. Insufficient funding could delay studies and prevent the establishment of necessary safeguards, which are vital for ensuring that research participants are treated ethically and that their safety is prioritized.
How do NIH research grants support patient safety in medical studies?
NIH research grants play a pivotal role in promoting patient safety in medical studies by providing essential resources for IRB oversight and ethical training. These grants help cover costs associated with the review and monitoring of clinical trials, which are essential for ensuring that researchers adhere to regulations designed to protect human subjects. The funding supports the infrastructure necessary to facilitate safe and ethical research practices across various institutions.
What role do IRBs have in safeguarding patients within the framework of medical research funding?
IRBs are integral to safeguarding patients in the realm of medical research funding. They review and oversee research proposals to ensure the protection of participants’ rights and welfare. With sufficient funding, IRBs can conduct thorough risk assessments, ensure informed consent processes are properly implemented, and monitor ongoing studies for compliance with ethical standards, ultimately fostering a secure environment for research participants.
What impact do cuts in medical research funding have on the ability to ensure informed consent?
Cuts in medical research funding can severely impact the ability to ensure informed consent in clinical studies. Limited resources can undermine the training of investigators on ethical practices and informed consent procedures, leading to potential gaps in participant understanding of study risks and benefits. This can compromise the integrity of the research process and diminish trust between researchers and participants.
How do institutional review boards (IRBs) facilitate multi-site research with medical research funding?
Institutional review boards (IRBs) facilitate multi-site research efficiency by operating under a single IRB (sIRB) model, a practice reinforced by NIH policies. Medical research funding is essential for supporting this model, allowing IRBs to manage oversight for studies conducted at multiple locations. This streamlines the approval process, reduces delays, and enhances collaboration among research institutions, ultimately benefiting patient safety and ethical compliance.
What are the long-term effects of disrupted medical research funding on public trust in healthcare research?
Disrupted medical research funding can lead to long-term erosion of public trust in healthcare research. When funding cuts halt or delay studies, especially those involving human participants, it can create skepticism and concern about the ethics and motives behind research practices. This mistrust can hinder future participation in clinical trials, ultimately impacting the advancement of medical knowledge and the development of new treatments.
Why is continuous medical research funding critical for improving patient safety protocols?
Continuous medical research funding is critical for improving patient safety protocols because it ensures that research institutions can uphold high standards of ethical oversight, training, and compliance. Funding allows for ongoing education for IRB members and researchers about evolving best practices in patient safety and protections. Without stable funding, research institutions may struggle to maintain these essential safety protocols, putting participants at risk.
| Key Point | Details |
|---|---|
| Impact of Funding Cuts | The Trump administration’s freeze of over $2 billion in federal grants to Harvard disrupts patient safety and oversight in medical research. |
| Role of IRBs | Institutional Review Boards (IRBs) ensure ethical oversight, protect participant welfare, and enforce compliance with laws and regulations in clinical research. |
| NIH Funding Importance | NIH funds vital programs aimed at protecting research participants, ensuring that projects receive rigorous oversight. |
| Consequences of Halted Studies | Halting studies not only risks participant safety but also exacerbates public mistrust in medical research, |
| Historical Context | Historical medical ethics violations underline the need for robust oversight provided by IRBs to prevent future harms. |
Summary
Medical research funding is crucial for ensuring the safety of patients involved in studies. The recent funding cuts have posed a significant risk to patient oversight and the ethical conduct of research. With institutions facing severe disruptions, the potential to protect participants and advance medical knowledge is severely hindered. To maintain participant safety and uphold ethical standards in research, it is imperative to ensure continuous and adequate medical research funding.